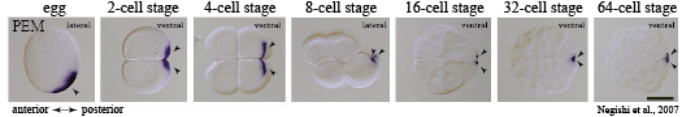
We have also found that PEM, like Drosophila Pgc and Caenorhabditis PIE-1, represses germline transcription by keeping RNAP II underphosphorylated
through binding to the p-TEFb complex (see Figure). Interestingly, PEM
is unique to ascidian species, and so are Pgc and PIE-1 to the Drosophila and Caenorhabditis species, respectively. Therefore, our study shows that three non-homologous proteins, PEM, Pgc, and PIE-1, may have been independently incorporated to play analogous roles through binding to p-TEFb. We believe that this is an interesting example of evolutionary constraint on how a mechanism of germline silencing can evolve in diverse animals and are trying to understand how it might have happened during evolution by also studying closely related species such as Ciona to Halocynthia. In addition, we continue to investigate to find out the functions of
other localized maternal factors in Halocynthia germline cell development, especially focusing on those in transcriptional
regulation. |
 |
 |
 |

Tail shaping
|
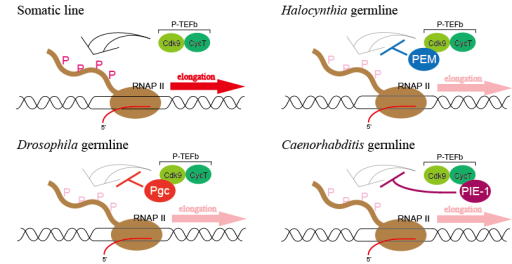
 In the ascidian late neurula embryo, the boundary between the trunk and
tail regions can be recognized morphologically for the first time in development
as a bending of the epithelial layer, which we call “KUBIRE” (a Japanese
word for Small waist, see red arrowheads in Figure). After the “KUBIRE”
formation, the posterior tail region elongates significantly and reaches
eventually four to five fold length of the trunk part. Although ascidians belong to the phylum Chordata as we human beings do,
the way of making tail in ascidians as mentioned above is quite different
from other chordates such as vertebrates and amphioxus in that they make
a cell mass called the tailbud at the tip of the neurula embryo and allow
it grow andextend posteriorly. In the ascidian late neurula embryo, the boundary between the trunk and
tail regions can be recognized morphologically for the first time in development
as a bending of the epithelial layer, which we call “KUBIRE” (a Japanese
word for Small waist, see red arrowheads in Figure). After the “KUBIRE”
formation, the posterior tail region elongates significantly and reaches
eventually four to five fold length of the trunk part. Although ascidians belong to the phylum Chordata as we human beings do,
the way of making tail in ascidians as mentioned above is quite different
from other chordates such as vertebrates and amphioxus in that they make
a cell mass called the tailbud at the tip of the neurula embryo and allow
it grow andextend posteriorly. |
 |
 |
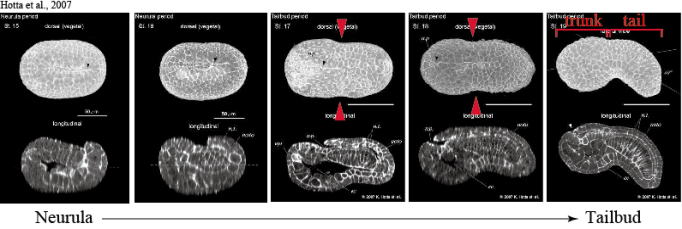
Therefore, we reasoned that we might be able to discover a new principle
for tissue shaping involved in an unusual way of making tail such as that
observed in the ascidian embryo. We are thus currently interested in elucidating
how and under what molecular basis individual cells move at right times
and right places during "KUBIRE" formation and how such cell
movements contribute to the tissue shaping.
|
 |
Branching morphogenesis |
|
| We are interested in branching morphogenesis of the medusa tentcles of
the jellyfish, Cladonema pacificum. One of the synapomorphic characteristics
in the family Cladonematidae is that the medusa tentacles are branched
with branches having nematocyst knobs and those having adhesive organs
for landing (see Figure). Therefore, firstly, comparative analyses of tentacle
branching mechanisms of Cladonema pacificum and other jellyfish species
could provide us a useful insight into the evolutionary process of the
acquisitions of a novel morphological trait (in this case, branching).
Secondary and more importantly, since jellyfishes are not well-studied
organisms and have simpe cell constitutions, we expect that studying their
branching morphogenesis might reveal a novel principle in tissue shaping
mechanisms, which could not be discovered in other branching phenomena
such as those in the fly trachea systems, the mammalian lungs and vertebrate
angiogenesis. |
 |
 |
|
 |
 |
|
|
|